Chemical reaction is a process in which one or more chemical substances are transformed into another one or two chemical substance. These chemical reactions can either be spontaneous or non-spontaneous. Chemical reaction is the name of transformation of one chemical substance to another and it doesn’t involve the creation or destruction of matter or energy. Chemical reactions are often grouped into four general categories which are given below:
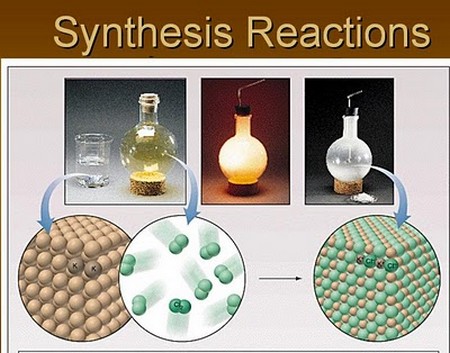
Synthesis Reaction
In this type of reaction, when two or more simple substances are combined, they form a more complex substance. In other words, when two reactants produce one product, it is known as synthesis reaction.
Example: When hydrogen is come in contact with oxygen, they yield a new complex substance, water. The chemical equation can be given as:
2H2 + O2 à 2H2O
Reactant + Reactant à Product
Decomposition Reaction
Decomposition reaction is a chemical reaction in which a more complex substance split into its more simple chemical substances. In other words, one reactant produces two or more products. Decompositions reactions are the exact opposite of synthesis reactions.
Example: When water is exposed to decomposition reaction, it decomposes into its simpler compounds, to say oxygen and hydrogen. The chemical equation of this reaction can be given as:
2H2O à 2H2 + O2
Reactant à Product + Product
Single Replacement Reaction
In this sort of chemical reaction, an uncombined single substance takes the place of another substance in a compound. In other words, two reactants produce two products.
Example: In zinc and hydrochloric acid reaction, the zinc takes the place of hydrogen. The chemical equation can be given as:
Zn + 2HCl à ZnCl2 + H2
Reactant + Reactant à Product + Product
Double Replacement Reaction
In this type of chemical reaction, switching is take place between parts of the chemical compounds to give rise to two new chemical compounds. In other words, two reactants produce two products.
Example: On reaction between silver nitrate and sodium chloride, two new chemical compounds are produced, i.e. silver chloride and sodium nitrate. In this case switching is take place between sodium and silver. The chemical reaction can be given as:
AgNO3 + NaCl à AgCl + NaNO3
Reactant + Reactant à Product + Product
Energy of Chemical Reactions
Whenever a chemical reaction occurs, energy is involved in it. Although, chemical reactions are not responsible for producing or destroying energy, but a lot of energy is absorbed or released during the chemical reactions. On the basis of energy, chemical reactions are classified as exothermic or endothermic reactions.
- Endothermic Reactions
Those reactions which require energy to complete are known as endothermic chemical reactions. The endothermic reactions usually absorb heat or electrical energy. Electrical energy helps to separate pure metal and oxygen from metal oxides. Similarly, electrical energy is used to split the sodium chloride in its original sodium and chlorine form.
- Exothermic Reactions
Those reactions which release energy are known as exothermic chemical reactions. The heat energy which is released during the chemical reaction is stored in chemical bonds. All those reactions which involve the combustion process come under exothermic chemical reactions.